Leveraging Technology to Improve Patient Recruitment for Clinical Trials
I was asked to respond to a costumer inquiry recently to help them with providing a solution to improve their patient recruitment in clinical trials. Initially, I thought this must be a question to one of our experts in our CRO. However, I kept thinking about it and realized there isn’t a silver bullet that would solve this problem. There is no commercial off-the-shelf solution to fix this problem. Sponsors, CROs, Site Coordinators, PIs and other stakeholders involved in the clinical research have been struggling to address this situation but it is still a challenge and is the root cause of delays in clinical trials which will result in increased and lower Returns on Investment (ROI).
Discovery and development of medical products take a long time and consume lot of resources (Effort & Cost) from sponsors from molecule to patient. This involves discovery of the molecule to pre-clinical and clinical development to marketing & sales. A lot of these resources are typically spent in late stage (Phase III) of clinical trials. One of the root causes for delays in clinical trials in phase III is delay in recruiting the patients that meet the inclusion criteria of the protocol. Another key concern is patient drop out during the course of the trial. These issues not only delay the trial but also force the sponsors and CROs to consider changes to the protocol or force them to take alternative steps to ensure the necessary patient population is recruited to continue the trial.
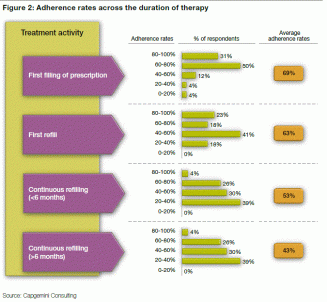
According to a report on Patient Adherence by Capgemini consulting [1], “Patient adherence levels vary between 50% for depression to 63% for enlarged prostate. On average, adherence levels drop over the course of the patient journey from 69% of patients filling their first prescription to 43% continuing their treatment as prescribed after 6 months.”
Also, if you notice in the attached diagram, 31% of the patients recruited will not last until they fill their prescription. Further, by the sixth month almost 57% patients do not show up for their refills. At this rate, in order to continue the trial, the sponsor has to continue to recruit patients during the course of the trial. This recruitment due to a drop out comes at a cost. The report has also identified that the cost of recruiting a new patient is 6 times the cost spent by sponsors to retain an existing patient.
So, what are the sponsors/CROs doing to improve the patient recruitment and reduce the drop outs? The key to fixing any problem is identifying the root cause. For this problem, there are multiple areas of failure and hence requires a comprehensive, multi-pronged strategy to fix the problems. Some of the best practices being adopted by sponsors and/or CROs include:
- Comprehensive and incremental approach to integrated solutions rather than silos by leveraging :
– Patient registries
– Better communication between patients and clinicians
– Electronic records
– Better engagement of physicians
- Refine Site selection
– Analysis to select better performing sites
- Patient centric recruitment process
– Simplify informed consent process (move to informed choice)
- Collaborative approach to Protocol design
– Engaging Principal Investigators (PIs) in protocol design by research coordinators
- Improve patient retention
– Better follow-up systems to increase adherence
– Enhanced post-trial engagement
In order to implement these best practices sponsors can adopt technology solutions like:
- Create strategy to improve the recruitment process by leveraging technology solutions
- Analyze site performance and select high performing sites and drop out non-performing sites
- Patient Recruitment Systems to search and match protocol eligibility criteria with patient’s available Electronic Health Records (EMRs, Narratives, Health Insurance and Claims Documents)
- Social Media tools to target patient communities to identify potential subjects from the available patient population
- Social Media tools to gauge trial buzz in patient and physician community
- Study & Investigator Portals to enhance engagement and collaboration between study coordinators and Principal Investigators
- Mobile Trial Adherence Systems to alert and notify patients of visits and other trial compliance activities to reduce drop outs
- Key Opinion Leader portals to better engage physicians
- Analytics to identify potential subjects, site initiation process and performance, patient recruitment effectiveness, patient drop out alerts etc.
- Advanced analytics to simulate recruitment performance based on historical data and thus tailoring recruitment strategy based on sites and other factors
Despite all these best practices and solutions, will be able to help the sponsors and CROs in:
- Collecting historical and real time operational and scientific data
- Performing objective analysis of data
- Visualization of such performance
- Identifying the bottlenecks and root causes for the problems
- Helping in making decisions to select the right sites, recruit the right patients, avoid costly drop outs and improve the collaboration and communication among Patients, Primary Investigators and other stakeholders.
I will be writing about each solution in my “Top 10” list above. As I mentioned in the beginning of this post, there is no silver bullet for this problem. A strategic and integrated approach to create solutions that will aid in collecting, analyzing and decision making is the only way to solve this problem.
As always, please let me know your feedback. If you have more insights into the problem or solutions, I will be more than glad to discuss with you and also post some of the inferences in a subsequent post, as promised. Happy Reading!!!
References:
- “Patient Adherence: The Next Frontier in Patient Care – Vision & Reality, 9th Edition Global Research Report” by Capgemini Consulting

The information in this article is really true, i agree with this article.AXIS Clinicals
Dear Venugopal,
You indicate companies use EHR data to help identify patient volunteers for clinical trials.
Would you be able to share specific examples?
Thanks in advance.
JC Muyl
Hi JC Muyl,
I am not at will to disclose the specific customer names. However, there have been many instances where Electronic Health Records have seen secondary uses (Pharmacovigilance, Phase IV Patient Recruitment, Outcomes Research etc.) in Life Sciences. A couple of white papers and articles that provide more information below:
Click to access us_lshc_Secondary%20Uses%20of%20EHR_0409.pdf
Click to access us_lshc_modernizingpharma_022410.pdf
Click to access Impact%20of%20re-use%20of%20Clinical%20Data_Pharmaceutical%20Industry%20Perspective_final.pdf
Hope this helps. I will be happy to discuss this offline, if you’d like.
Cheers,
Venu.